The answer for the following problem is mentioned below.
- Therefore number of molecules(N) present in the calcium phosphate sample are 19.3 × 10^23 molecules.
Step-by-step explanation:
Given:
mass of calcium phosphate (
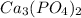 ) = 125.3 grams
) = 125.3 grams
We know;
molar mass of calcium phosphate (
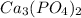 ) = (40×3) + 3 (31 +(4×16))
) = (40×3) + 3 (31 +(4×16))
molar mass of calcium phosphate (
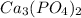 ) = 120 + 3(95)
) = 120 + 3(95)
molar mass of calcium phosphate (
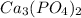 ) = 120 +285 = 405 grams
) = 120 +285 = 405 grams
We also know;
No of molecules at STP conditions(
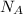 ) = 6.023 × 10^23 molecules
) = 6.023 × 10^23 molecules
To solve:
no of molecules present in the sample(N)
We know;
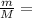 N÷
N÷
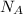
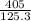 =
=
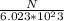
N =(405×6.023 × 10^23) ÷ 125.3
N = 19.3 × 10^23 molecules
Therefore number of molecules(N) present in the calcium phosphate sample are 19.3 × 10^23 molecules