Answer:
1.99 x 10²³ formula units
Step-by-step explanation:
Given parameters:
Mass of AgF = 42.15g
Unknown:
The amount of formula units
Solution:
To solve this problem, we set out by find the number of moles in this compound from the given mass.
Number of moles =

molar mass of AgF = 107.9 + 19 = 126.9g/mol
Number of moles =
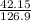 = 0.33 moles
= 0.33 moles
1 mole of a substance = 6.02 x 10²³ formula units
0.33 moles of AgF = 0.33 x 6.02 x 10²³ = 1.99 x 10²³ formula units