Option C
The average rate of the reaction over the entire course of the reaction is:
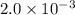
Solution:
Average rate is the ratio of concentration change to the time taken for the change
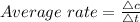
The concentration of the reactants changes 1.8 M to 0.6 M
here, the time interval given is 0 to 580 sec
Therefore,
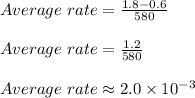
Thus option C is correct