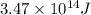The question is incomplete, here is the complete question:
A nuclear fisston reaction has mass difference between the products and the reactants of 3.86 g. Calculate the amount of energy released by the reaction.
A) 1.16 * 10^6 * J
B) 2.59 * 10^9
C) 3.47 * 10^ 14 * J
D) 4.82 * 10^17 J
Answer: The amount of energy released by the reaction is
Step-by-step explanation:
To calculate the energy released, we use Einstein equation, which is:

where,
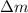 = mass defect = 3.86 g = 0.00386 kg (Conversion factor: 1 kg = 1000 g)
= mass defect = 3.86 g = 0.00386 kg (Conversion factor: 1 kg = 1000 g)
c = speed of light =
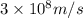
Putting values in above equation, we get:
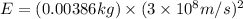
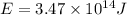
Hence, the amount of energy released by the reaction is