Answer: The law that illustrates this is Boyle's Law
Step-by-step explanation:
- Boyle's law: This law states that pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
Mathematically,
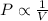 (at constant temperature and number of moles)
(at constant temperature and number of moles)
- Charles' law: This law states that volume of the gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
Mathematically,
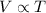 (at constant pressure and number of moles)
(at constant pressure and number of moles)
- Avogadro's law: This law states that volume of the gas is directly proportional to the number of moles of the gas at constant pressure and temperature.
Mathematically,
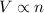 (at constant pressure and temperature)
(at constant pressure and temperature)
So, when the volume of the air in the gun decreases, the pressure is increased. The inverse relationship between the volume and pressure of the gas is very well illustrated by Boyle's Law.
Hence, the law that illustrates this is Boyle's Law