Answer:
0.24mole
Step-by-step explanation:
Given parameters:
Number of molecules = 14.35 x 10²² molecules;
Unknown = number of moles of H₃PO₄
A mole of a substance is the quantity of matter that contains the avogadro's number of particles.
This number is given as 6.02 x 10²³ particles. The particles can be atoms, molecules, protons, electrons e.t.c
6.02 x 10²³ molecules is contained in 1 mole of any substance
14.35 x 10²² molecules will contain:
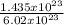 = 0.24mole
= 0.24mole