Answer:
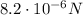 , attractive
, attractive
Step-by-step explanation:
The magnitude of the electrostatic force between two charges is given by Coulomb's law:
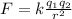
where:
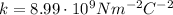 is the Coulomb's constant
is the Coulomb's constant
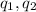 are the two charges
are the two charges
r is the separation between the two charges
And the force is:
- Repulsive if the two charges have same sign
- Attractive if the two charges have opposite signs
In this problem:
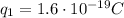 is the magnitude of the charge of the electron
is the magnitude of the charge of the electron
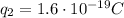 is the magnitude of the charge of the proton
is the magnitude of the charge of the proton
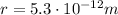 is the separation between the two particles
is the separation between the two particles
So the magnitude of the force is
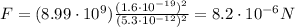
And since the two charges have opposite signs, the force is attractive.