Answer:
The metal will have changed temperature more than the water.
Step-by-step explanation:
As we know that when two objects are mixed at different temperature then due to heat exchange between the two they both reach to same final temperature
This is known as thermal equilibrium
So here we have
Heat given by metal = heat absorbed by water
so we have
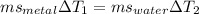
since mass of metal is same as that of water
so we have
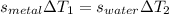
so here we know that
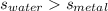
so temperature change in water must be smaller than that of metal