Answer:
About 0.164 g of glucose.
Step-by-step explanation:
We can determine the mass of glucose produced given the volume of oxygen gas produced with stoichiometry.
Recall that at STP, a mole of any gas occupies a volume of 22.4 L.
From the reaction, six moles of oxygen gas is produced for every one mole of glucose.
Lastly, the molecular weight of glucose is 180.18 g/mol.
Therefore:
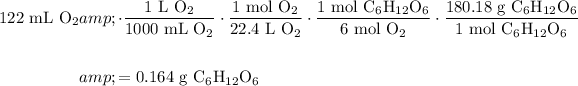
Therefore, about 0.164 g of glucose is produced.