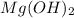Answer: In the given acid-base reaction, the base is
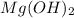
Step-by-step explanation:
Acids are defined as the chemical species which donate hydrogen ions when dissolved in water.
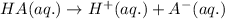
Bases are defined as the chemical species which donate hydroxide ions when dissolved in water.
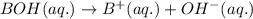
The given chemical equation follows:
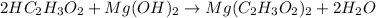
Here, magnesium hydroxide is acting as base because it dissociates into magnesium ions and hydroxide ions when dissolved in water.
The chemical equation for the ionization of magnesium hydroxide follows:
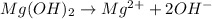
Hence, in the given acid-base reaction, the base is