Answer:
About 2.29 M.
Step-by-step explanation:
Recall that molarity is defined by moles of solute over liters of solution.
Our solute is 105 grams of calcium nitrate (Ca(NO₃)₂)).
Convert 105 grams of Ca(NO₃)₂ to moles. The molecular weight of Ca(NO₃)₂ is 164.10 g/mol:
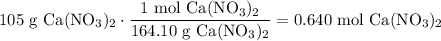
Convert 280. grams of water to liters. Recall that the density of water is given by 1.00 g/mL:
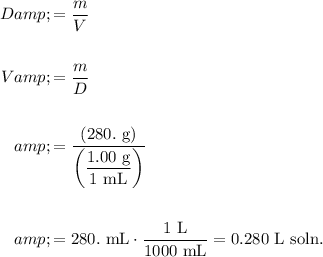
Hence, the molarity of the solution is:
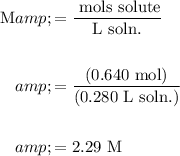
In conclusion, the solution is about 2.29 M.