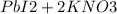Answer:
Explanation:
insoluble salts are prepared by the reaction of two soluble salts.
To prepare lead iodide, react aqueous solutions of KI and Pb(NO3)2, you'll get yellow precipitate of lead iodide and soluble potassium nitrate. separate the mixture by filtration. wash the residue (PbI2) and dry.
see equation below
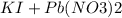 →
→