Answer:
The half life time of first order reaction is 1.3 sec
Step-by-step explanation:
Given:
First order rate constant
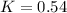
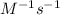
Initial concentration
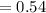 M
M
From the formula of first order half life time,
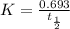
So half life time is given by,
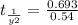
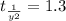 sec
sec
Therefore, the half life time of first order reaction is 1.3 sec.