Answer:
0.42 g
Step-by-step explanation:
We have:
pH = 12.10 (25 °C)
V = 800.0 mL = 0.800 L
To find the mass of sodium hydroxide (NaOH) we can use the pH:
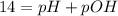
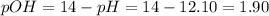
![pOH = -log ([OH^(-)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/vbncg7i88ubudzhl80atygonaxm2pygsn4.png)
![[OH]^(-) = 10^(-pOH) = 10^(-1.90) = 0.013 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/hjckde60k73jypcooke3i8jehdh59c2741.png)
Now, we can find the number of moles (η) of OH:
![\eta = ([OH]^(-))*V = 0.013 mol/L * 0.800 L = 1.04 \cdot 10^(-2) moles](https://img.qammunity.org/2021/formulas/chemistry/high-school/14yi40yct8gcjzp2pmfao5y29opm64bdvj.png)
Since we have 1 mol of OH in 1 mol of NaOH, the number of moles of NaOH is equal to 1.04x10⁻² moles.
Finally, with the number of moles we can find the mass of NaOH:
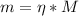
Where M is the molar mass of NaOH = 39.9 g/mol
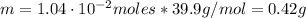
Therefore, the mass of sodium hydroxide that the chemist must weigh out in the second step is 0.42 g.
I hope it helps you!