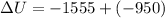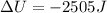Answer:
The change on the internal energy of the system is -2505 joules
Step-by-step explanation:
To solve this problem, we're going to use the first law of thermodynamics, that it's essentially a law of conservation of energy. It states that the change on the internal energy of a system is the sum of the work (performed on the system or performed by the system) and the heat the system exchange with the surroundings (could be entering on the system or leaving the system), mathematically:
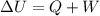
with ΔU the change on the internal energy, Q the heat and W the work.
As the question say the gun imparts on the potato 950 joules of work, that means the work is done by the gone so by convention it's negative. The heat released is 1555 joules and because is leaving the system is negative too, then: