Answer:
Efficiency of the engine is 39.51 %
Step-by-step explanation:
Mass of fuel consumed per hour = 22 L/h * 0.08 Kg/L = 17.6 Kg/h
Total Energy Consumed per hour = Mass of fuel consumed per hour * Heating value of fuel
Total Energy Consumed per hour = 17.6 Kg/h * 44,000 KJ/Kg = 774,400 KJ/h
since, 1 KW = 3600 KJ/h
774,400KJ/h = 774,400/3600 kW = 215.1 kW
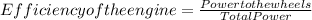
Efficiency = 85/215.1 = 39.51 %