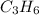The molecular formula for the compound is
Step-by-step explanation:
As with all of these problems, we assume 100 g of an unknown compound.
And thus, we determine the elemental composition by the given percentages.
Moles of carbon = 85.64 / 12.011
= 7.13 mol.
Moles of hydrogen = 14.36 / 1.00794
= 14.25 mol.
There are 2 moles of hydrogen per mole of carbon. And thus the empirical formula is CH
 .
.
And molecular formula = n × (empirical formula)
Thus, 42.08 = n × (12.011 + 2 × 1.00794)
And thus n = 3, and molecular formula =