Answer:
1.484 grams.
Step-by-step explanation:
At first we have to find the number of moles in 0.0350 M of 800.0 mL of Na solution. For this we will use the molarity equation as follows -
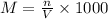
Where,
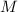 = molarity of the solution
= molarity of the solution
 = number of moles of Na
= number of moles of Na
 = volume of solution in mL
= volume of solution in mL
From the above equation,
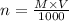 =
=
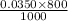 = 0.028 moles.
= 0.028 moles.
Now, we know that sodium carbonate dissociates as
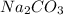 ⇒
⇒
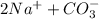
That means 1 mole of sodium carbonate produces 2 moles of Na⁺ ion
So, the moles of sodium carbonate required for 0.028 mole of Na⁺ ion will be
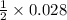 = 0.014 moles.
= 0.014 moles.
So, the weight of sodium carbonate required = number of moles × molecular mass
= 0.014 × 105.9888 = 1.484 g
The weight of sodium carbonate required for preparing the solution mentioned in the question is 1.484 grams.