Answer: The molar concentration of Z in the solution is 2.15 M
Step-by-step explanation:
We are given:
23% (w/w) Z in solution
This means that 23 grams of Z is present in 100 grams of solution
To calculate the volume of solution, we use the equation:
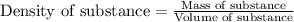
Density of solution = 1.3 g/mL
Mass of solution = 100.0 g
Putting values in above equation, we get:
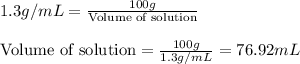
To calculate the molarity of solution, we use the equation:
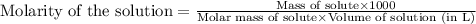
Given mass of Z = 23 g
Molar mass of Z = 139 g/mol
Volume of solution = 76.92 mL
Putting values in above equation, we get:

Hence, the molar concentration of Z in the solution is 2.15 M