Answer:
The structure analysis says the compound must be Cumene or isopropylbenzene
Step-by-step explanation:
Degree of unsaturation or double bond equivalent
D.B.E =
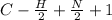
=
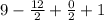
= 4
¹H NMR data analysis
(i) 1.2δ (doublet, I = 6H) two CH₃ are equivalents and the multiplicity says the neighboring carbon have one hydrogen.
(ii) 3.0δ (septet, I = 1H), one CH and the multiplicity says the neighboring carbon have six hydrogens.
(iii) 7.1δ (multiplet, I = 5H) , means
and the sturcture of the compound is