concluding part of the question
Determine;
a.) the temperature at the end of each heat addition process in K
b.) the net work of the cycle per unit mass of air in kJ/kg
c.) the thermal efficiency
Answer:
(a)
 = 731.3 K,
= 731.3 K,
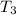 = 1865.8 K,
= 1865.8 K,
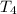 = 2266.67 K and
= 2266.67 K and
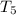 = 1145.75 K
= 1145.75 K
(b) the net work of the cycle per unit mass of air = 828.41 kJ/kg
(c) the thermal efficiency = 55.23%
Step-by-step explanation:
Given;
compression ratio
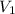 /
/
 =10
=10
Heat per unit mass
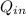 /m = 1500 kJ/kg
/m = 1500 kJ/kg
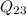 /m = 1000 kJ/kg
/m = 1000 kJ/kg
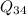 /m = 500 kJ/kg
/m = 500 kJ/kg
 = 100 kPa
= 100 kPa
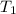 = 300 K
= 300 K
Assumptions:
1. the air in piston cylinder assembly is the closed system
2. all processes are internally reversible
3. the air is modeled as an ideal gas.
4. the compression and expansion processes are adiabatic.
5. kinetic and potential energy effect are negligible.
Note: analysis of the cycle is done by fixing each principle state of the cycle.
State 1:
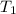 = 300 K ⇒
= 300 K ⇒
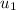 = 214.07 kJ/kg,
= 214.07 kJ/kg,
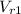 = 621.2
= 621.2
State 2: for isentropic compression
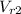 =
=
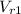 *
*
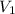 /
/
 = 621.2/10 = 62.12
= 621.2/10 = 62.12
thus,
 = 731.3 K and
= 731.3 K and
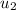 = 535.6 kJ/kg
= 535.6 kJ/kg
State 3: for the heat addition process from 2 to 3.
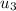 =
=
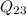 /m +
/m +
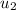 = 1000 + 535.6 = 1535.6 kJ/kg
= 1000 + 535.6 = 1535.6 kJ/kg
(a) thus,
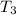 = 1865.8 K and
= 1865.8 K and
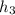 = 2070.52 kJ/kg
= 2070.52 kJ/kg
State 4: for the heat addition process from 3 to 4
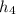 =
=
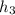 +
+
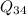 /m = 2070.52 + 500 = 2570.52 kJ/kg
/m = 2070.52 + 500 = 2570.52 kJ/kg
thus,
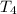 = 2266.67 K and
= 2266.67 K and
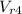 = 2.013
= 2.013
State 5: for isentropic expansion
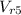 =
=
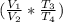
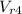 = ((10 * (1865.8/2266.67)) = 16.57
= ((10 * (1865.8/2266.67)) = 16.57
thus,
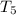 = 1145.75 K and
= 1145.75 K and
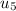 = 885.66 kJ/kg
= 885.66 kJ/kg
(b)
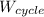 =
=
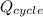 , thus
, thus
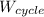 /m =
/m =
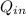 /m - (
/m - (
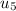 -
-
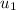 ) = 1500 - (885.66 - 214.07) = 828.41 kJ/kg
) = 1500 - (885.66 - 214.07) = 828.41 kJ/kg
(c) the thermal efficiency η
η =
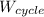 /m/
/m/
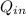 /m = 828.41/1500 = 0.552 (55.23%)
/m = 828.41/1500 = 0.552 (55.23%)