Answer:
- To increase the temperature as it is a reactant in terms of its endothermicity.
- To remove it will enable more space for the reactant to favor its production.
- To add more reactant in order to increase its equilibrium concentration.
Step-by-step explanation:
Hello,
The undergoing chemical reaction is:
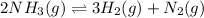
Thus, in order to intensify the amount of nitrogen as the chemical reaction is endothermic, considering the Le Chatelier's principle we state:
- To increase the temperature as it is a reactant in terms of its endothermicity.
- To remove it will enable more space for the reactant to favor its production.
- To add more reactant in order to increase its equilibrium concentration.
Best regards.