Answer with Explanation:
Ionic bond:It is that type of bond which formed between negative and positive ions.
Hydrogen bonding:Hydrogen bonding formed between hydrogen and high electronegative elements.
Dispersion force:It is weakest intermolecular force.It is temporary attractive force.When electrons of two adjacent atoms occupy positions that make the atoms temporary dipoles.
NaCl: In which Na is positive ion and Cl is negative ion.
Therefore, NaCl is ionic.
HF:F is highest electronegativ elements.
HF:Hydrogen bonding
HCl: Hydrogen bonding
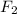 :Dispersion
:Dispersion