Answer:
4.5 moles of carbon dioxide
Step-by-step explanation:
Given parameters:
Mass of CH₄ = 72g
Unknown:
number of moles of CO₂ produced = ?
Solution:
To solve this problem, let us write the reaction equation first;
CH₄ + 2O₂ → CO₂ + 2H₂O
Now we know that O₂ is in excess and CH₄ is the limiting reactant that will determine the extent of the reaction.
Let us find the number of moles of CH₄ ;
Number of moles =
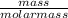
molar mass of CH₄ = 12 + 4 = 16g/mol
Number of moles =
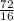 = 4.5moles
= 4.5moles
From the reaction equation;
1 mole of methane produced 1 mole of carbon dioxide
4.5 moles of methane will produce 4.5 moles of carbon dioxide