Answer:
18.5 degree Celsius
Step-by-step explanation:
We are given that
Partial pressure of gas=
 =72.92 KPa
=72.92 KPa
Total pressure gas =P=75.02 KPa
We have to find the temperature at which the gas was collected.
We know that
Total pressure,P=
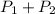
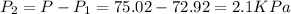
Water vapor pressure=
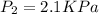
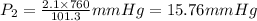
101.3 KPa=760 mm Hg
Temperature when water vapor pressure is 15.75 mm Hg=18.5 degree Celsius
The temperature at which gas collected=18.5 degree Celsius