Answer :
(1) A is reactant.
(2) B is reactant.
(3) C is product.
(4) D is product.
(5) (s) represents solid state.
(6) (l) represents liquid state.
(7) (g) represents gaseous state.
(8) (aq) represents aqueous state.
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
The species present on the left side of the right arrow is the reactant and the species present on the right side of the right arrow is the product.
Some symbols used in the chemical reaction are :
The symbol
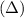 is used for temperature.
is used for temperature.
The symbol (s) for solid, (l) for liquid, (g) for gaseous and (aq) for aqueous are used for the representation of physical states.
The given chemical reaction is:
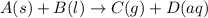
In this reaction,
A and B are reactants.
C and D are products.
(s) represents solid state.
(l) represents liquid state.
(g) represents gaseous state.
(aq) represents aqueous state.