Answer:
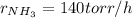
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
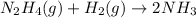
Thus, in terms of pressures, the rate becomes:
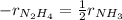
Thus, the rate of change for the partial pressure of ammonia turns out:
![r_(NH_3)=2*(-r_(N_2H_4))\\r_(NH_3)=2*[-(-70torr/h)]\\r_(NH_3)=140torr/h](https://img.qammunity.org/2021/formulas/chemistry/college/dhc1imzd7d0xb1xcs6pkkx9zskcls4gf5c.png)
The rate of decrease of partial pressure of urea is taken negative as it is a reactant whereas ammonia a product which has 2 as its stoichiometric coefficient.
Best regards.