Answer:
Approximately
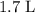 .
.
Step-by-step explanation:
Nitrogen
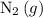 reacts with hydrogen
reacts with hydrogen
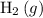 at a
at a
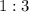 ratio to produce ammonia
ratio to produce ammonia
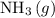 :
:
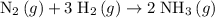 .
.
The ratio between the coefficient of
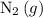 and the coefficient of
and the coefficient of
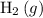 is:
is:
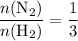 .
.
Under the ideal gas assumptions, the same ratio would apply to the volume of
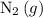 and
and
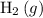 in this reaction:
in this reaction:
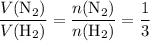 .
.
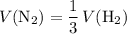 .
.
Given that
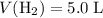 :
:
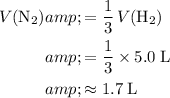 .
.
(Rounded to
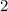 significant figures.)
significant figures.)