0.2625 litres is the volume of methanol should be used to make 1.25 L of a 21.0% by volume solution.
Step-by-step explanation:
Given that:
Volume of solution = 1.25 litres
methanol to be present in the composition of 21% of the volume.
To make 21 % solution by volume of a substance it means that 21 L of the substance is present in 100 L of the solution which is made upto 100 L by adding the solvent.
So, 21 L in 100 L will make 21% solution
x litres of methanol in 1.25 L of solution
So,
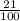 =
=
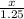
x = 0.2625 litres
hence, 0.2625 litres of the methanol will be added and the volume will be made to 1.25 litres so that 21% solution by volume gets form.