Answer: The given reaction is non-spontaneous at low temperatures and spontaneous at high temperatures.
Step-by-step explanation:
We know that relation between free energy and enthalpy is as follows.
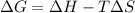
As in the given drawing, solid is converting into gas. This means that there is occurring an increase in entropy of the system. This also means that the enthalpy change will also be positive.
Free energy change is negative (spontaneous) at high temperature and free energy change is positive at low temperature.
Hence, we can conclude that the given reaction is non-spontaneous at low temperatures and spontaneous at high temperatures.