Answer:
V = 23515.53volt
Step-by-step explanation:
wavelength λ= 1.1 x 10 -11 m
We know λ= h /√ [2mVq]
Where h = Plancks constant
= 6.625 x 10 -34 J s
m = mass of electron
= 9.11 x 10 -31 kg
q = charge of eectron
=1.6 x 10 -19 C
From above potential difference V = ( 1/2mq) (h /λ)²
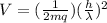
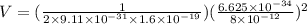
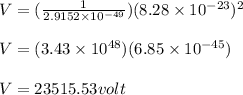
V = 23515.53volt