Answer:
0.31M
Step-by-step explanation:
Given parameters:
Mass of NaNO₃ = 130g
Volume of solution = 5L
Molar mass of NaNO₃ = 23 + 14 + 3(16) = 85g/mol
Unknown:
Molarity of solution = ?
Solution:
Molarity is one of the ways of expressing concentration.
It is defined as the number of moles in a volume of solution.
Molarity =
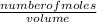
let us find the number of moles of NaNO₃;
Number of moles =
 =
=
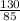 = 1.53moles
= 1.53moles
Molarity of solution =
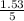 = 0.31M
= 0.31M