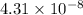This is an incomplete question, here is a complete question.
A 0.130 mole quantity of NiCl₂ is added to a liter of 1.20 M NH₃ solution. What is the concentration of Ni²⁺ ions at equilibrium? Assume the formation constant of Ni(NH₃)₆²⁺ is 5.5 × 10⁸
Answer : The concentration of
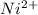 ions at equilibrium is,
ions at equilibrium is,
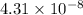
Explanation : Given,
Moles of
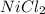 = 0.130 mol
= 0.130 mol
Volume of solution = 1 L
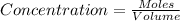
Concentration of
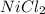 = Concentration of
= Concentration of
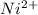 = 0.130 M
= 0.130 M
Concentration of
 = 1.20 M
= 1.20 M
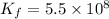
The equilibrium reaction will be:
![Ni^(2+)(aq)+6NH_3(aq)\rightarrow [Ni(NH_3)_6]^(2+)](https://img.qammunity.org/2021/formulas/chemistry/college/76v6n9lu7nvflhhgtdm7t8v2guzs568xxu.png)
Initial conc. 0.130 1.20 0
At eqm. x [1.20-6(0.130)] 0.130
= 0.42
The expression for equilibrium constant is:
![K_f=([Ni(NH_3)_6^(2+)])/([Ni^(2+)][NH_3]^6)](https://img.qammunity.org/2021/formulas/chemistry/college/on515ya7oa3jrrgjpckvhwntufs7asamdn.png)
Now put all the given values in this expression, we get:
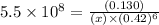
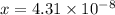
Thus, the concentration of
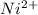 ions at equilibrium is,
ions at equilibrium is,