Answer:
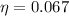
Step-by-step explanation:
Given that
Temperature of warm water ,T₁ = 25 °C
Temperature of cold water ,T₂ = 5 °C
The maximum possible efficiency of any heat is will be same as the efficiency of the Carnot heat engine.Therefore the maximum efficiency of OTEC system is given as follows
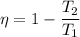
T should be Kelvin scale
Now by putting the values
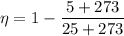
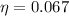
Therefore the maximum efficiency will be 0.067 or 6.7%.