Answer:
a) The detailed explanation for the question being asked in section a is shown below
b)

Equilibrium constant expression
![K_c = ([HSO_3^-][OH^-])/(]SO_3^(2-)])](https://img.qammunity.org/2021/formulas/chemistry/college/k6j2zgwpzetw93zwo1611ncsj39xkasec0.png)
c) pH = 10.45
Step-by-step explanation:
a.
If we look at
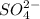 , we will realize that it is a conjugate base of a strong acid
, we will realize that it is a conjugate base of a strong acid
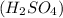 . However, the more stronger the acid, the weaker its conjugate base.
. However, the more stronger the acid, the weaker its conjugate base.
On the other hand
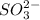 is a conjugate base of a weak acid
is a conjugate base of a weak acid
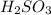 and the more weaker the acid, the stronger the basicity of its conjugate base.
and the more weaker the acid, the stronger the basicity of its conjugate base.
b. The chemical equation for the reaction between
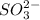 and water can be expressed as follows:
and water can be expressed as follows:

Equilibrium constant
![K_c = ([HSO_3^-][OH^-])/(]SO_3^(2-)])](https://img.qammunity.org/2021/formulas/chemistry/college/k6j2zgwpzetw93zwo1611ncsj39xkasec0.png)
c.
The ICE Table is then constructed as follows:

Initial (M) 0.500 0 0
Change (M) - x + x + x
Equilibrium (M) (0.500 - x) x x
![K_c = ([HSO_3^-][OH^-])/(]SO_3^(2-)])](https://img.qammunity.org/2021/formulas/chemistry/college/k6j2zgwpzetw93zwo1611ncsj39xkasec0.png)
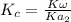
where
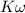 is the ionic product of water =
is the ionic product of water =
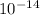
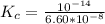
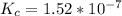
However;
![1.52*10^(-7) = ([x][x])/([0.500 - x])](https://img.qammunity.org/2021/formulas/chemistry/college/ef7w1vxy5h2933jz7hhzx4nn9rh48flhlm.png)
![1.52*10^(-7) = ([x]^2)/([0.500])](https://img.qammunity.org/2021/formulas/chemistry/college/tdecli7om8yq8mqpwn19793q8mmpvg9eht.png) since
since
 value is so small; then (0500 -x ) ≅ 0.500
value is so small; then (0500 -x ) ≅ 0.500

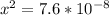

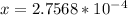
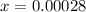
![[OH^-] = x = 0.00028](https://img.qammunity.org/2021/formulas/chemistry/college/yfnnsnmrmfpstba18cpc5ieme65zmywphk.png)
![pOH = -log \ [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/y8oxexta2lmikwyc5oen462ocn25d30edx.png)
![pOH = - log \ [0.00028]](https://img.qammunity.org/2021/formulas/chemistry/college/f1mk8x5e4ehlhpwtxjdfb89i51cv649b2o.png)
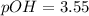
pH + pOH = 14
pH + 3.55 = 14
pH = 14 - 3.55
pH = 10.45