Answer:
- Empirical:
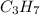
- Molecular:
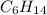
Step-by-step explanation:
Hello,
In this case, based on the information regarding the combustion, the moles of carbon turn out:
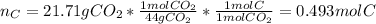
Moreover, the moles of hydrogen:
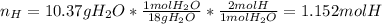
Thus, the subscripts of carbon and hydrogen in the hydrocarbon turn out:
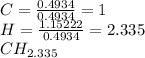
Now, looking for a suitable whole number we obtain the following empirical formula as 2.335 times 3 is 7 for hydrogen:
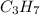
In such a way, that compound has a molar mass of 43 g/mol, thus, the whole compound's molar mass is 86.18 g/mol for which the molecular formula is twice the empirical one, therefore:
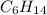
Which is hexane.
Best regards.