Answer:
The new pressure (atm) = 14.7 atm or 11172 torr
Step-by-step explanation:
P₁V₁ = P₂V₂ (using Boyle's law at constant temperature)
Volume of sample of gas collected in the upper atmosphere (V₁) = 35.0 Lit
pressure of the gas (P₁) = 48.6 torr
1 atm = 760 torr
⇒
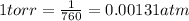
⇒ 48.6 torr = 48.6 x 0.00131 = 0.063 atm
Volume after compression (V₂) = 150 ml = 0.15 lit
New pressure (P₂) = ?
P₁V₁ = P₂V₂
⇒ P₂ =
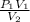
⇒ P₂ =
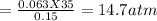 or 11172 torr
or 11172 torr