Answer:
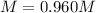
Step-by-step explanation:
Hello,
In this case, it is firstly necessary to compute the dissolved moles of hydrogen chloride into the water as shown below:
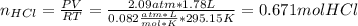
Thus, the molarity is computed as shown below:
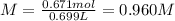
Wherein no change in volume is considered, therefore the volume of the solution was the same volume of water.
Best regards.