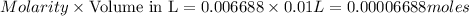Answer: The concentration of
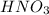 in the final solution is 0.006688 M and number of moles are 0.00006688
in the final solution is 0.006688 M and number of moles are 0.00006688
Step-by-step explanation:
According to the neutralization law,
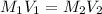
where,
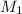 = molarity of stock solution = 6.847 M
= molarity of stock solution = 6.847 M
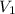 = volume of stock solution = 25.00 ml
= volume of stock solution = 25.00 ml
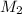 = molarity of ist dilute solution = ?
= molarity of ist dilute solution = ?
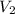 = volume of first dilute solution = 100.0 ml
= volume of first dilute solution = 100.0 ml
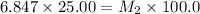
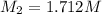
2) on second dilution;
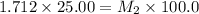
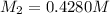
3) on third dilution
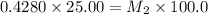
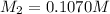
4) on fourth dilution
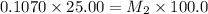
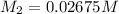
5) on fifth dilution
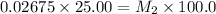
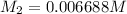
Thus the concentration of
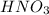 in the final solution is 0.006688 M
in the final solution is 0.006688 M
moles of
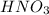 =
=