Answer: The concentration of hydrogen gas at equilibrium is 0.037 M
Step-by-step explanation:
We are given:
Initial concentration of HI = 1.0 M
The given chemical equation follows:
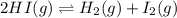
Initial: 1.0
At eqllm: 1.0-2x x x
The expression of
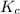 for above equation follows:
for above equation follows:
![K_c=([H_2][I_2])/([HI]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/eomp0tp40lrrfz3unwe6kx0agfhigvm2po.png)
We are given:
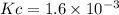
Putting values in above expression, we get:
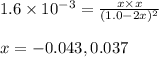
Neglecting the negative value of 'x' because concentration cannot be negative
So, equilibrium concentration of hydrogen gas = x = 0.037 M
Hence, the concentration of hydrogen gas at equilibrium is 0.037 M