Answer:
0.0253 M/s
Step-by-step explanation:
From the reaction
N₂ + 3H₂ → 2NH₃
The rate of reaction can be written as
Rate = -
![(d[N_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/z99ptgv0k70rgz4b8w61rbulfxce3g6gil.png) = -
= -
![(1)/(3) (d[H_2])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/nbuouev0nr28xlucox5cm5tq8gbcamnz75.png) = +
= +
![(1)/(2) (d[NH_3])/(dt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/yj4w7sb9ckq8ra0avqz4ruu9udpi4rdb5c.png)
From the above rate equation we can conclude that the rate of reaction of N₂ is equal to one third of the rate of reaction of H₂,
So,
Rate of reaction of molecular nitrogen =
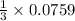
Upon calculation, we get rate of reaction of molecular nitrogen = 0.0253 M/s