Answer : The mass percent and molality of ethanol is, 8.28 % and 1.96 mol/kg respectively.
Explanation :
As we are given that 10.5 % ethanol by volume. That means, 10.5 mL of ethanol present in 100 mL of solution.
Density of ethanol =
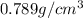
First we have to calculate the mass of ethanol.
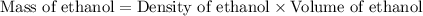
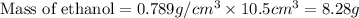
Now we have to calculate the volume of water.
Volume of water = Volume of solution - Volume of ethanol
Volume of water = (100 - 8.28) cm³
Volume of water = 91.7 cm³
Now we have to calculate the mass of water.
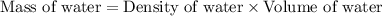
Density of water = 1.00 g/cm³
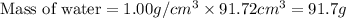
Total mass = 8.28g + 91.7g = 99.98 g
Now we have to calculate the mass percent of ethanol.
Mass percent of ethanol =
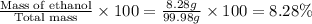
Now we have to calculate the Moles of ethanol.
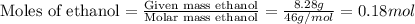
Now we have to calculate the molality of ethanol.
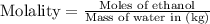
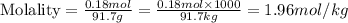
Therefore, the mass percent and molality of ethanol is, 8.28 % and 1.96 mol/kg respectively.