Answer:
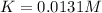
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction turns out:
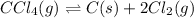
In such a way, by means of the mass of law action for such reaction, which is given below:
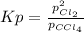
And in terms of the change
 due to reaction extent:
due to reaction extent:
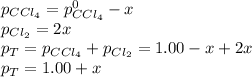
 results:
results:
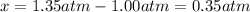
In such a way, Kp:
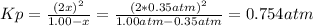
Nonetheless, K is asked instead of Kp, thus:
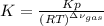
Whereas:
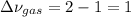
Which is the change in the moles of gaseous species chlorine and carbon tetrachloride. Hence, we finally obtain:
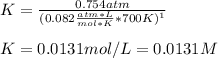
Best regards.