Answer: The mass of zinc chloride produced in the reaction is 54.1 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
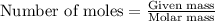 .....(1)
.....(1)
Given mass of zinc = 26 g
Molar mass of zinc = 65.4 g/mol
Putting values in equation 1, we get:
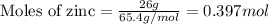
Given mass of HCl = 42 g
Molar mass of HCl = 36.5 g/mol
Putting values in equation 1, we get:
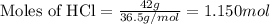
The chemical equation for the reaction of zinc and HCl follows:
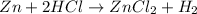
By Stoichiometry of the reaction:
1 mole of zinc reacts with 2 moles of HCl
So, 0.397 moles of zinc will react with =
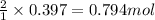 of HCl
of HCl
As, given amount of HCl is more than the required amount. So, it is considered as an excess reagent.
Thus, zinc metal is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
1 mole of zinc produces 1 mole of zinc chloride
So, 0.397 moles of zinc will produce =
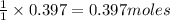 of zinc chloride
of zinc chloride
Now, calculating the mass of zinc chloride from equation 1, we get:
Molar mass of zinc chloride = 136.3 g/mol
Moles of zinc chloride = 0.397 moles
Putting values in equation 1, we get:
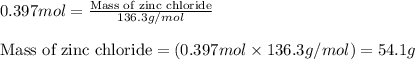
Hence, the mass of zinc chloride produced in the reaction is 54.1 grams