Answer:
the free electron density of lithium is 2.3 × 10²⁸ /m³
Step-by-step explanation:
The Fermi energy is 4.72 eV.
The Fermi energy (highest filled orbital energy ) of free electron is
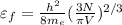
Rearrange the above equation for free electron density of lithium
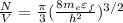
where,
h = 6.626 × 10⁻³⁴J.s
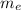 = 9.11 × 10⁻³¹kg
= 9.11 × 10⁻³¹kg
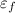 = 4.72eV
= 4.72eV
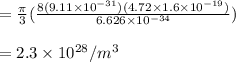
Thus, the free electron density of lithium is 2.3 × 10²⁸ /m³