Answer: When an atom gains an electron to achieve stability, it becomes negatively charged. The charge on an atom becomes positive when it loses (donates) an electron.
Step-by-step explanation:
When a neutral atom tends to lose an electrons then due to decrease in number of electrons holded by the atom there will occur a positive charge on the atom. For example, electronic distribution of calcium is 2, 8, 8, 2.
So, in order to attain stability it will lose its 2 valence electrons and hence becomes
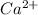 ion.
ion.
This also means that charge on the atom increases. And, when a neutral atom loses an electron then due to increase in number of electrons into the atom there will occur a negative charge on the atom.
For example, electronic distribution of oxygen is 2, 6. To attain stability it will gain 2 electrons and hence, it formed
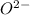 ion.
ion.
Thus, we can conclude that when an atom gains an electron to achieve stability, it becomes negatively charged. The charge on an atom becomes positive when it loses (donates) an electron.