Answer:
The volumetric flow rate of air through the heat exchanger is 3.36 m³/s.
Step-by-step explanation:
Here we have,
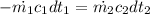
The properties of R 134a at 700 kPa Saturated temperature = 26.7 °C and enthalpy = 88.8 kJ/kg
While at super heated temperature and pressure of 70 kPa and 50 °C the enthalpy is 288.53 kJ/kg
Therefore, we have, the heat lost per kg = 288.53 kJ/kg - 88.8 kJ/kg = 199.73 kJ/kg
For air we have at 20 ° and 100 kPa, enthalpy = 294 kJ/kg
At 25 °
 = 1.012 J·g⁻¹·K⁻¹
= 1.012 J·g⁻¹·K⁻¹
20 ° C
 = 1.006 kJ/(kg·K)
= 1.006 kJ/(kg·K)
Therefore,
 air = 1.006 kJ/(kg·K)
air = 1.006 kJ/(kg·K)
Plugging in the values into the above equation, we have
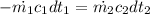 = 0.099 kg/sec × 199.73 kJ/kg =
= 0.099 kg/sec × 199.73 kJ/kg =
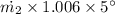
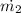 = 19.77/5.03 = 3.93 kg/s
= 19.77/5.03 = 3.93 kg/s
At 100 kPa and 25° C the density of air is 1.16843 kg/m³
Therefore the volume flow rate = 3.93/1.16843 =3.36 m³/s.