Answer: The value of K for this reaction is 1.6875
Step-by-step explanation:
Moles of
 = 4.0 mole
= 4.0 mole
Volume of solution = 2.0 L
Initial concentration of
 =
=
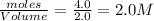
The given balanced equilibrium reaction is,
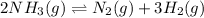
Initial conc. 2 M 0 M 0 M
At eqm. conc. (2-2x) M (x) M (3x) M
Equilibrium concentration of
 =
=
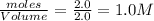
The expression for equilibrium constant for this reaction will be,
![K_c=([x]* [3x]^3)/([(2-2x)]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/5m6kivaxcn089pl04uk34nws38tu5m7qm1.png)
(2-2x) = 1.0
x= 0.5 M
Now put all the given values in this expression, we get :
![K_c=([0.5]* [3* 0.5]^3)/([(2-2* 0.5)]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/146h97row2ynqdxmhy8v1nnc55azqyvhwe.png)
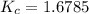
Thus the value of K for this reaction is 1.6875