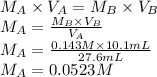Answer:
A. 0.143 M
B. 0.0523 M
Step-by-step explanation:
A.
Let's consider the neutralization reaction between potassium hydroxide and potassium hydrogen phthalate (KHP).
KOH + KHC₈H₄O₄ → H₂O + K₂C₈H₄O₄
The molar mass of KHP is 204.22 g/mol. The moles corresponding to 1.08 g are:
1.08 g × (1 mol/204.22 g) = 5.28 × 10⁻³ mol
The molar ratio of KOH to KHC₈H₄O₄ is 1:1. The reacting moles of KOH are 5.28 × 10⁻³ moles.
5.28 × 10⁻³ moles of KOH occupy a volume of 36.8 mL. The molarity of the KOH solution is:
M = 5.28 × 10⁻³ mol / 0.0368 L = 0.143 M
B.
Let's consider the neutralization of potassium hydroxide and perchloric acid.
KOH + HClO₄ → KClO₄ + H₂O
When the molar ratio of acid (A) to base (B) is 1:1, we can use the following expression.