Answer:
0.01744 M is the hardness of the groundwater.
1,744 ppm is the hardness of the groundwater in parts per million of calcium carbonate by mass.
Step-by-step explanation:
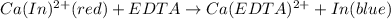
In = Indicator
Concentration of calcium ion in groud water =
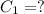
Volume of ground water =
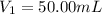
Concentration of EDTA solution =
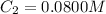
Volume of EDTA solution =
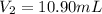
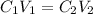
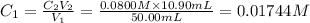
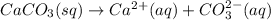
![[CaCO_3]=[Ca^(2+)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/41mwv0ds7jcop2e2lt1ad9p27kex8s1je1.png)
So.
![[CaCO_3]=0.01744 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/412p9rogzlf0zb3twcoe5w97y7p4z9rktc.png)
0.01744 M is the hardness of the groundwater.
0.01744 Moles of calcium carbonate are present in 1 Liter of solution.
Mass of 0.01744 moles of calcium carbonate =
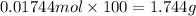
1 g = 1000 g
1.744 g = 1.744 × 1000 mg = 1,744 mg
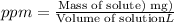
The hardness of the groundwater in parts per million :
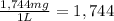
1,744 ppm is the hardness of the groundwater in parts per million of calcium carbonate by mass.